-
US | en

Fluorescence
When a fluorophore absorbs light, its electrons become excited and move from a resting state (S0, Figure 8A) to a maximal energy level called the excited electronic singlet state (S2) (1). The amount of energy required for this transition will differ for each fluorophore. The duration of the excited state depends on the fluorophore and typically lasts for 1-10 nanoseconds. The fluorophore then undergoes a conformational change, the electrons fall to a lower, more stable energy level called the electronic singlet state (S1), and some of the absorbed energy is released as heat (2). The electrons subsequently fall back to their resting state (S0) releasing the remaining energy (EEmission) as fluorescence (3). The difference between wavelengths of the emission and excitation maxima is called the Stokes shift (Figure 8B). This cycle can repeat several thousand times for a single fluorophore, which allows recycling of fluorophores and thus amplification of the signal.
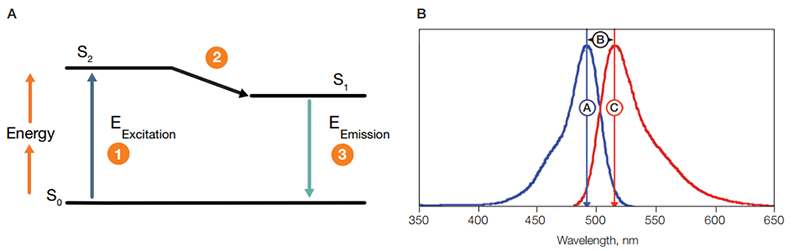
Fig. 8. Stokes shift. A, upon excitation, 1, electrons in a fluorophore move from a resting state, S0 , to the excited electronic single state, S2. Some energy is released as heat, 2. The remaining energy is released as fluorescence, 3, as the electrons return to their ground state, S0. B, the difference between the excitation maxima,
 , and the emissions maxima,
, and the emissions maxima,
 , of a fluorophore is called its Stokes shift
, of a fluorophore is called its Stokes shift
 .
.
Emitted light typically contains less energy than was originally put into the fluorophore to excite it. Therefore, the emission wavelength of any fluorophore is longer (lower energy) than its excitation wavelength and thus a different color.
The wavelength of excitation is critical to the total photons of light that the fluorophore will absorb. Fluorescein isothiocyanate (FITC), for example, will absorb light from 400-530 nm but absorbs most efficiently at its peak or excitation maximum of 490 nm wavelength. It is desirable to excite fluorophores at their excitation maximum because the more photons are absorbed, the more intense the fluorescence emission will be. The wavelengths of greatest absorption and emission are termed maximal absorbance and maximal emission wavelengths.
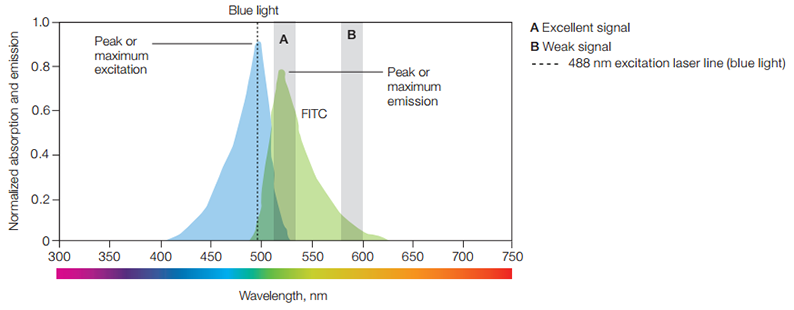
A fluorophore's maximal absorbance informs you which laser line is optimal to be used for excitation. In the case of FITC, its maximum absorbance falls within the blue spectrum. Therefore, the blue 488 nm laser, which is close to FITC’s absorbance peak of 490 nm, is commonly used to excite this fluorophore. FITC emits fluorescence from 475 to 650 nm, peaking at 525 nm, which falls in the green spectrum. How the flow cytometer is set up determines how the fluorophore is detected. If the filters are used to screen out all light other than that measured at the maximum absorbance via channel A (Figure 9), FITC will appear green. Fluorescence color usually refers to the color of light a fluorophore emits at its highest stable excited state.
Setup determines perception
However, if FITC fluorescence is detected only via channel B (Figure 9), it will appear orange and be much weaker in intensity. How the flow cytometer is set up to measure fluorescence will thus ultimately determine the perceived color of a fluorophore. Because the color of the exciting and emitting light is different, they can be separated from one another by using optical filters.
