CD146 antibody | OJ79c












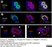




Mouse anti Human CD146
- Product Type
- Monoclonal Antibody
- Clone
- OJ79c
- Isotype
- IgG1
- Specificity
- CD146
| Mouse anti Human CD146 antibody, clone OJ79c recognizes human Cell surface glycoprotein MUC18, also known as CD146, Cell surface glycoprotein P1H12, Melanoma cell adhesion molecule (MCAM) or S-endo 1 endothelial-associated antigen. CD146 is a 646 amino acid single pass type 1 transmembrane glycoprotein with a calculated molecular mass of ~72 kDa. However due to extensive N-linked glycosylation CD146 migrates in polyacrylamide gels with an apparent molecular mass of ~118 kDa. CD146 is a member of the immunoglobulin superfamily bearing 2 V-type Ig-like and 3 C-type Ig-like domains. CD146 is expressed by all endothelial cells and by melanoma cells and appears to act as an adhesion molecule (UniProt: P43121). Expression in melanoma may be linked to tumor progression (Lehmann et al. 1989). Mouse anti Human CD146 antibody, clone OJ79c is highly expressed on pericytes and has been utilized for the identification of perivascular mesenchymal precursor cells from cardiac muscle using flow cytometry (Chen et al. 2014). |
- Target Species
- Human
- Species Cross-Reactivity
-
Target Species Cross Reactivity Pig Dog - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Recombinant human MUC18 (D1-D5) Fc protein.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunized mice were fused with cells of the mouse Sp2/0 Ag.14 myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA | |||
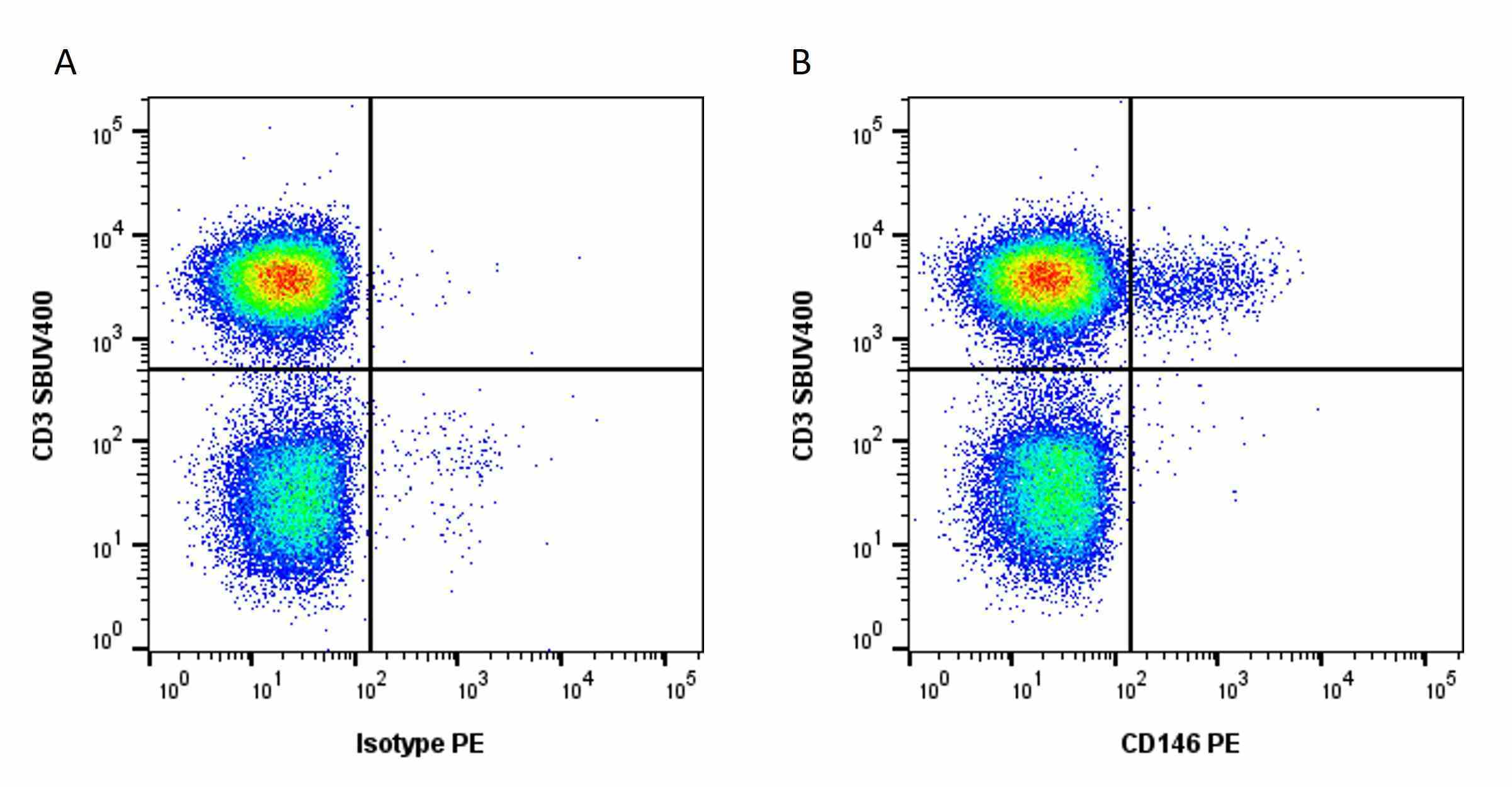
| Flow Cytometry | 1/10 | 1/50 | |
| Immunofluorescence | |||
| Immunohistology - Frozen |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
- Histology Positive Control Tissue
- Melanoma
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control | MCA928 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control | ||||||
References for CD146 antibody
-
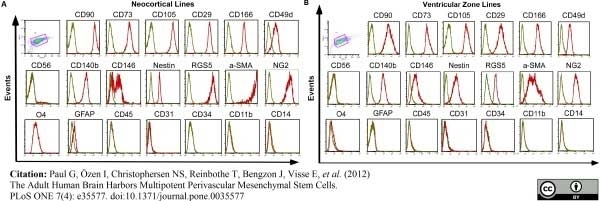
Paul, G. et al. (2012) The adult human brain harbors multipotent perivascular mesenchymal stem cells.
PLoS One. 7: e35577. -
Crisan, M. et al. (2008) A perivascular origin for mesenchymal stem cells in multiple human organs.
Cell Stem Cell. 3: 301-13. -
Iohara, K. et al. (2008) A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp.
Stem Cells. 26 (9): 2408-18. -
Park, T.S. et al. (2010) Placental Perivascular Cells for Human Muscle Regeneration.
Stem Cells Dev. 20: 451-63. -
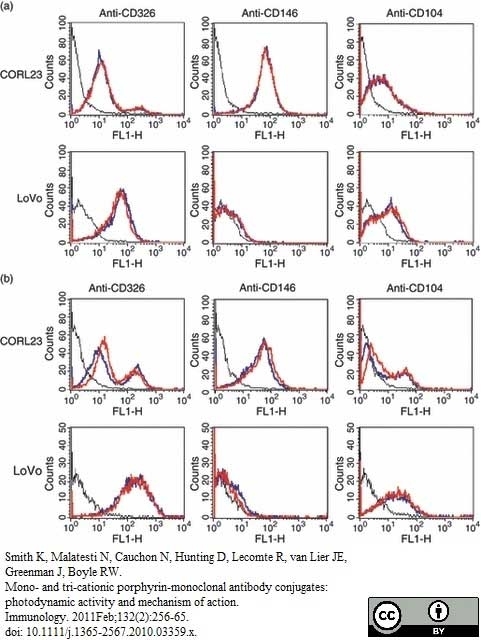
Smith, K. et al. (2011) Mono- and tri-cationic porphyrin-monoclonal antibody conjugates: photodynamic activity and mechanism of action.
Immunology. 132: 256-65. -
James, A.W. et al. (2012) Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering.
Stem Cells Transl Med. 1 (6): 510-9. -
Wassmer, S.C. et al. (2011) Vascular endothelial cells cultured from patients with cerebral or uncomplicated malaria exhibit differential reactivity to TNF.
Cell Microbiol. 13: 198-209. -
Lee, J.H. et al. (2012) Generation of osteogenic construct using periosteal-derived osteoblasts and polydioxanone/pluronic F127 scaffold with periosteal-derived CD146 positive endothelial-like cells.
J Biomed Mater Res A.101: 942-53.
View The Latest Product References
-
Boneberg, E.M. et al. (2009) Soluble CD146 is generated by ectodomain shedding of membrane CD146 in a calcium-induced, matrix metalloprotease-dependent process.
Microvasc Res. 78: 325-31. -
Nielsen, C.T. et al. (2011) Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus.
Arthritis Rheum. 63: 3067-77. -
Iversen, L.V. et al. (2013) A heparin-based method for flow cytometric analysis of microparticles directly from platelet-poor plasma in calcium containing buffer.
J Immunol Methods. 388 (1-2): 49-59. -
Meireles, A.L. et al. (2011) Increased levels of circulating endothelial progenitor cells in human T-cell lymphotropic virus type I carriers.
Arch Med Res. 42: 34-7. -
Iversen, L.V. et al. (2013) Circulating microparticles and plasma levels of soluble E- and P-selectins in patients with systemic sclerosis.
Scand J Rheumatol. 42 (6): 473-82. -
Murakami, M. et al. (2013) The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential.
Biomaterials. pii: S0142-9612(13)00942-3. -
Ruetze, M. et al. (2013) A novel niche for skin derived precursors in non-follicular skin.
J Dermatol Sci. 69: 132-9. -
Dokić, J. et al. (2013) Mesenchymal stem cells from periapical lesions modulate differentiation and functional properties of monocyte-derived dendritic cells.
Eur J Immunol. 43: 1862-72. -
Chen, W.C. et al. (2015) Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity.
Stem Cells. 33 (2): 557-73. -
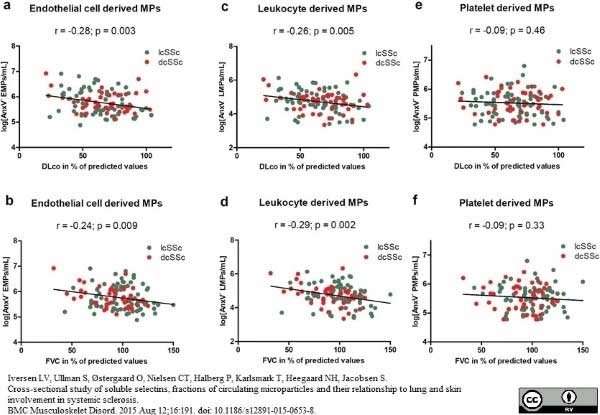
Iversen, L.V. et al. (2015) Cross-sectional study of soluble selectins, fractions of circulating microparticles and their relationship to lung and skin involvement in systemic sclerosis.
BMC Musculoskelet Disord. 16: 191. -
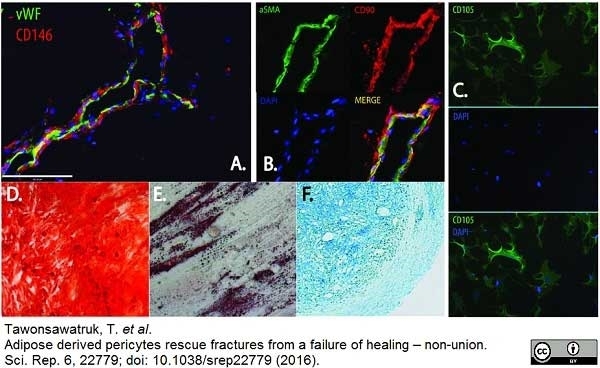
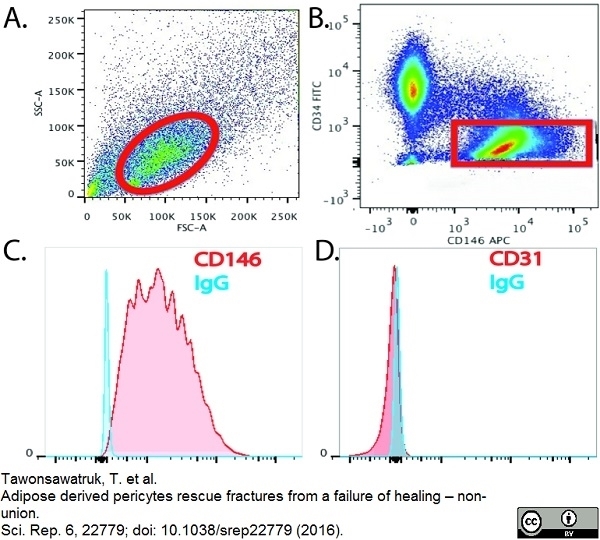
Tawonsawatruk T et al. (2016) Adipose derived pericytes rescue fractures from a failure of healing - non-union.
Sci Rep. 6: 22779. -
Boissier, R. et al. (2016) Histologic and urodynamic effects of autologous stromal vascular fraction extracted from fat tissue with minimal ex vivo manipulation on a porcine model of intrinsic sphincter deficiency
J Urology. Jun 2 [Epub ahead of print] -
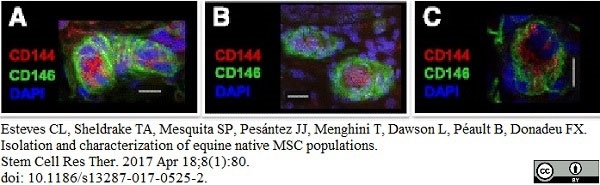
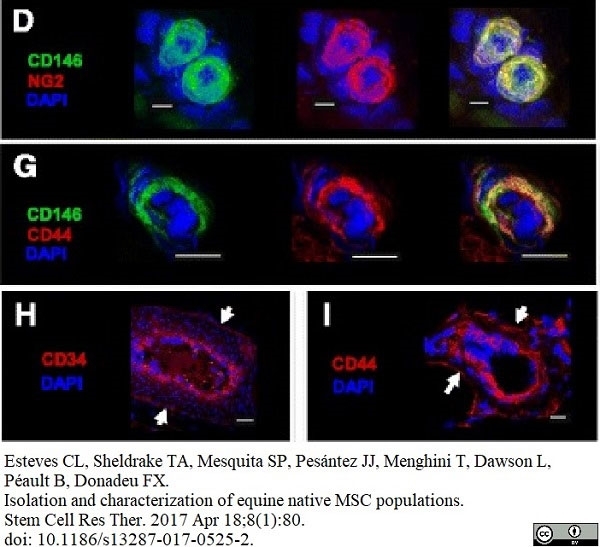
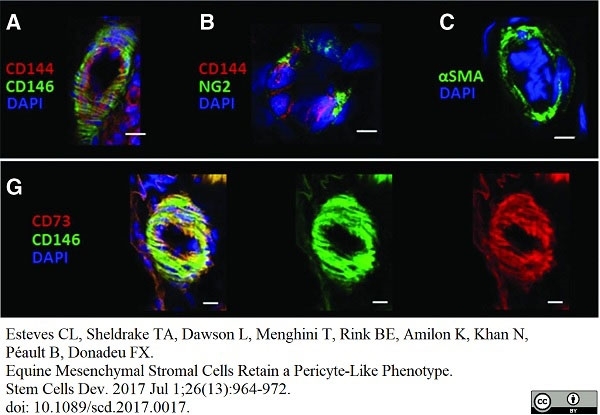
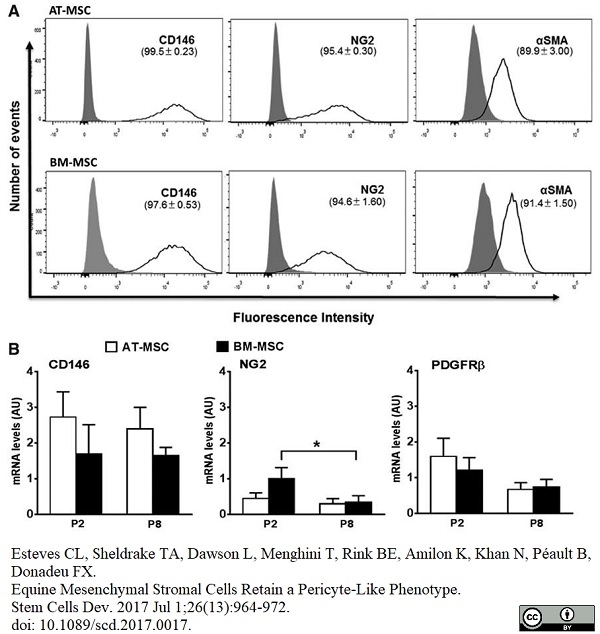
Esteves, C.L. et al. (2017) Equine Mesenchymal Stromal Cells Retain a Pericyte-Like Phenotype.
Stem Cells Dev. 26 (13): 964-72. -
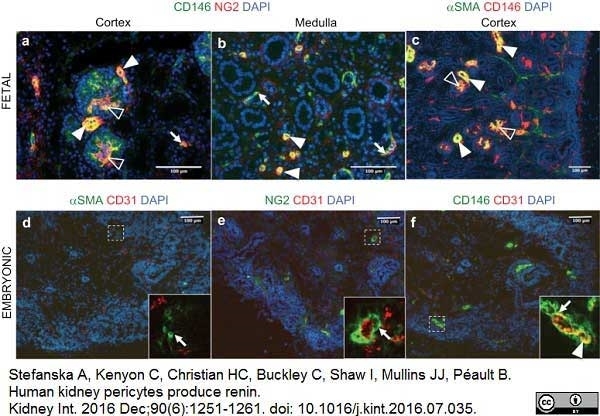
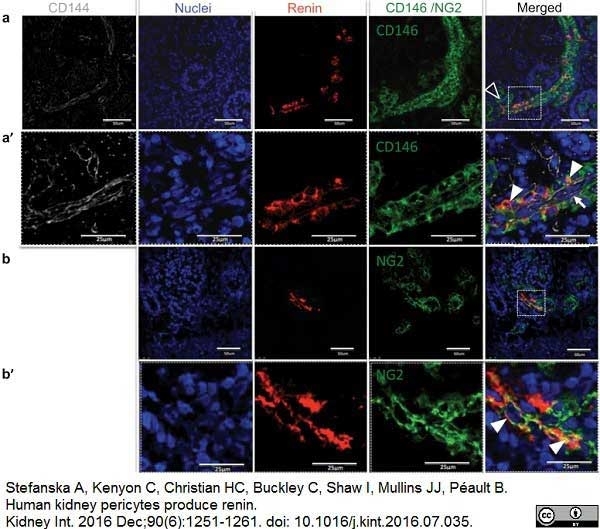
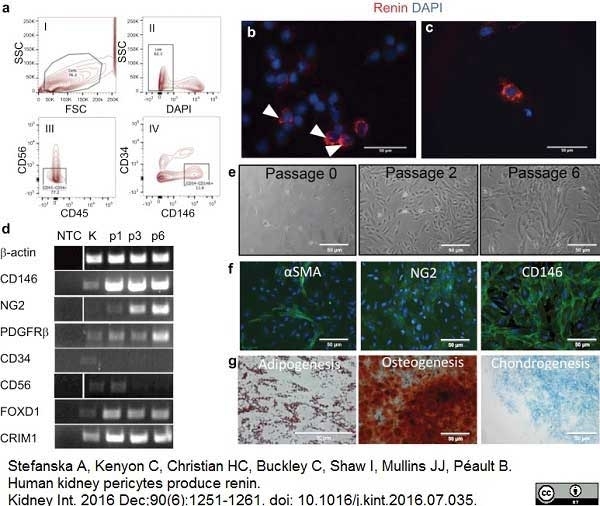
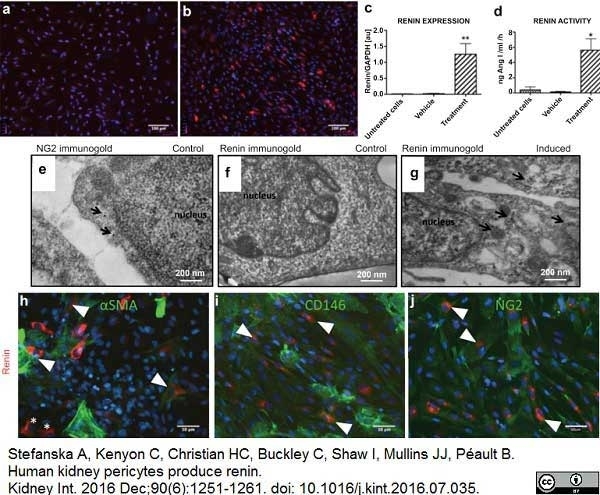
Stefanska, A. et al. (2016) Human kidney pericytes produce renin.
Kidney Int. 90 (6): 1251-61. -
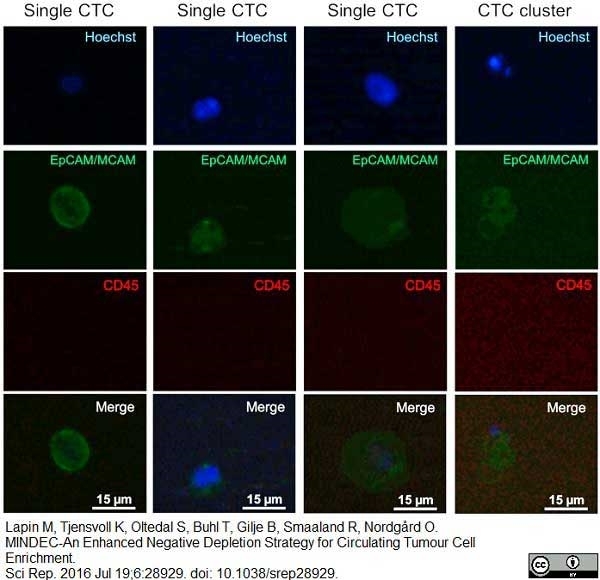
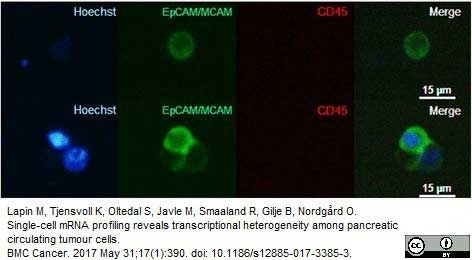
Lapin, M. et al. (2016) MINDEC-An Enhanced Negative Depletion Strategy for Circulating Tumour Cell Enrichment.
Sci Rep. 6: 28929. -
Muerza-Cascante, M.L. et al. (2016) Endosteal-like extracellular matrix expression on melt electrospun written scaffolds.
Acta Biomater. pii: S1742-7061(16)30706-1. -
Eliasberg, C.D. et al. (2017) Perivascular Stem Cells Diminish Muscle Atrophy Following Massive Rotator Cuff Tears in a Small Animal Model.
J Bone Joint Surg Am. 99 (4): 331-41. -
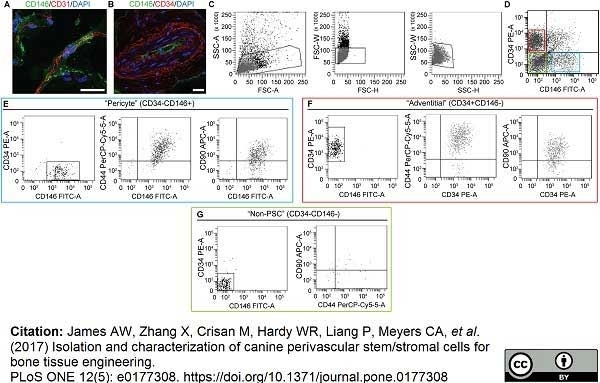
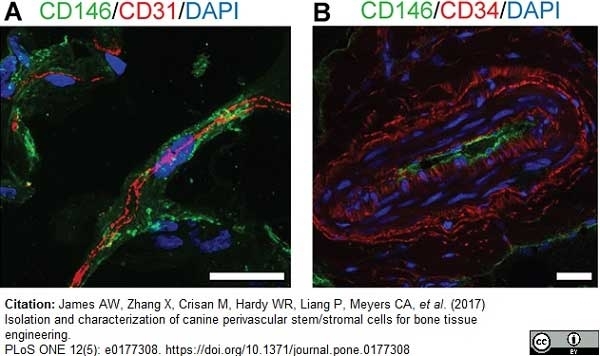
James, A.W. et al. (2017) Isolation and characterization of canine perivascular stem/stromal cells for bone tissue engineering.
PLoS One. 12 (5): e0177308. -
Shen, J. et al. (2018) Effects of WNT3A and WNT16 on the Osteogenic and Adipogenic Differentiation of Perivascular Stem/Stromal Cells.
Tissue Eng Part A. 24 (1-2): 68-80. -
Lapin, M. et al. (2017) Single-cell mRNA profiling reveals transcriptional heterogeneity among pancreatic circulating tumour cells.
BMC Cancer. 17 (1): 390. -
Esteves, C.L. et al. (2017) Isolation and characterization of equine native MSC populations.
Stem Cell Res Ther. 8 (1): 80. -
Gaceb, A. et al. (2017) Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB.
J Cereb Blood Flow Metab. : 271678X17719645. -
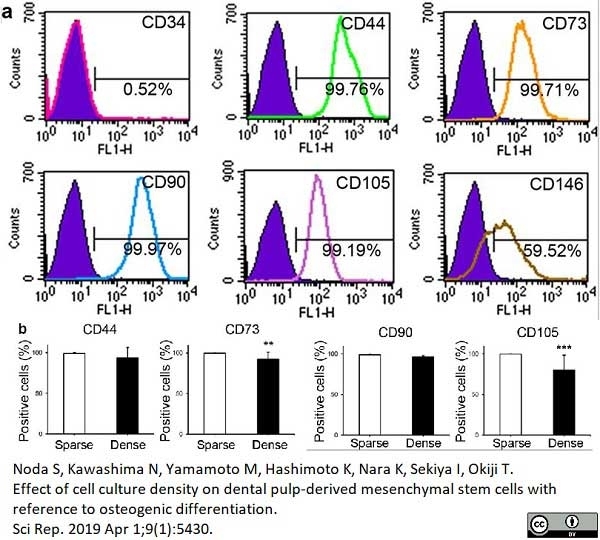
Noda, S. et al. (2019) Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation.
Sci Rep. 9 (1): 5430. -
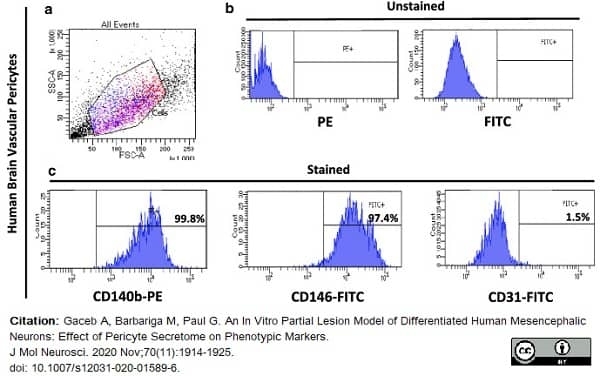
Gaceb, A. et al. (2020) An In Vitro. Partial Lesion Model of Differentiated Human Mesencephalic Neurons: Effect of Pericyte Secretome on Phenotypic Markers.
J Mol Neurosci. 70 (11): 1914-25. -
Stefanska, A. et al. (2021) Role of Pericytes in the Development of the Renin/Angiotensin System: Induction of Functional Renin in Cultures of Pericytes.
Methods Mol Biol. 2235: 169-180. -
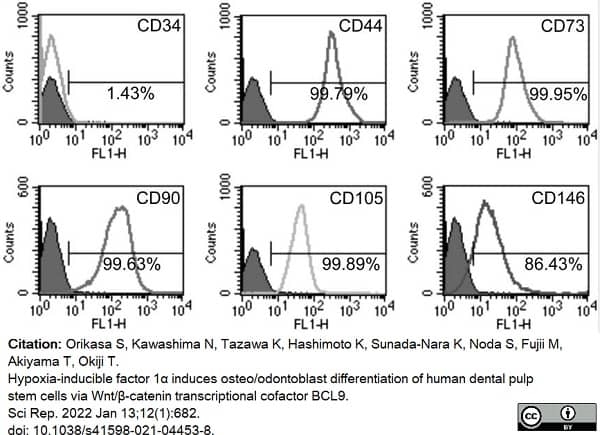
Orikasa, S. et al. (2022) Hypoxia-inducible factor 1α induces osteo/odontoblast differentiation of human dental pulp stem cells via Wnt/β-catenin transcriptional cofactor BCL9.
Sci Rep. 12 (1): 682. -
Menon, R. et al. (2023) Human Induced Pluripotent Stem Cell-Derived Pericytes as Scalable and Editable Source to Study Direct Lineage Reprogramming Into Induced Neurons.
Cell Reprogram. 25 (5): 212-23. -
Molinos, M. et al. (2023) Alterations of bovine nucleus pulposus cells with aging.
Aging Cell. 22 (8): e13873. -
An, H.J. et al. (2021) Pro-Angiogenic and Osteogenic Effects of Adipose Tissue-Derived Pericytes Synergistically Enhanced by Nel-like Protein-1.
Cells. 10 (9): 2244.
Further Reading
-
Kuzu, I. et al. (1993) Expression of adhesion molecules on the endothelium of normal tissue vessels and vascular tumors.
Lab Invest. 69 (3): 322-8. -
Piriou-Guzylack, L. (2008) Membrane markers of the immune cells in swine: an update.
Vet Res. 39: 54.
- Synonyms
- MUC18
- RRID
- AB_324068
- UniProt
- P43121
- Entrez Gene
- MCAM
- GO Terms
- GO:0005886 plasma membrane
- GO:0007155 cell adhesion
- GO:0016021 integral to membrane
- GO:0009653 anatomical structure morphogenesis
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Human ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up