
Popular topics

-
References
Blandino R and Baumgarth N. (2020). Secreted IgM: new tricks for an old molecule. J Leukoc Biol 106, 1,021-1,034.
Hiramoto E et al. (2018). The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Sci Adv 4, eaau1199.
Hughey CT et al. (1998). Production of IgM hexamers by normal and autoimmune B cells: implications for the physiologic role of hexameric IgM. J Immunol 161, 4,091-4,097.
Janeway CA Jr et al. (2001). The distribution and functions of immunoglobulin isotypes. In Immunobiology: the immune system in health and disease. 5th edition. (New York, Garland Science).
Oskam N et al. (2023). CD5L is a canonical component of circulatory IgM. Proc Natl Acad Sci USA 120, e2311265120.
Schroeder HW and Cavacini L. (2013). Structure and function of immunoglobulins. J Allergy Clin Immunol 125, S41-52.
Wei H and Wang JY. (2021). Role of polymeric immunoglobulin receptor in IgA and IgM transcytosis. Int J Mol Sci 22, 2,284.
Zhang J et al. (2022). Therapeutic antibodies for COVID-19: is a new age of IgM, IgA and bispecific antibodies coming? MAbs 14, 2031483.
The Past, the Present, and the Future of Immunoglobulin M

Upon stimulation of the B cell receptor (BCR) with its cognate antigen, B cells become activated and differentiate into long-lived, antibody-producing plasma cells to initiate the humoral immune response. Antibodies come in five main isotypes, as classified by the type of heavy chain they express: immunoglobulin (Ig) M, IgG, IgA, IgD, and IgE. IgM is the first class of antibody expressed during B cell development and in response to antigen challenge (Schroeder and Cavacini 2013). IgM was first characterized back in 1944, and since then, a plethora of studies have focused on further illuminating its role (Blandino and Baumgarth 2019).
So, given the mountain of research performed on this molecule, you might imagine its structure would be textbook knowledge. Well, researchers at Utrecht University and Sanquin recently made discoveries that may challenge that notion (Oskam et al. 2023).
The Past
What do we know about IgM up to now? The reason IgM is the first antibody produced is because it does not require isotype switching. This is a process that usually occurs after activation of the B cell, which allows it to change its isotype to the most productive to eliminate the pathogen while retaining its antigen specificity. Early IgM antibodies are also produced before somatic hypermutation of the B cell has occurred, which usually facilitates the development of high-affinity antibodies. As a result, IgM antibodies are often of low affinity.
However, they overcome this pitfall by forming pentamers, comprising a total of ten heavy (H) chains, ten light (L) chains, and one joining (J) chain (Figure 1). Importantly, these pentamers will also have ten antigen-binding sites, thereby overcoming the low affinity of IgM molecules by bestowing high avidity (Janeway et al. 2001). It has also been observed that if IgM is expressed without the J-chain, it can form hexamers. However, so far this has been associated with pathologies such as autoimmune diseases, rather than under normal conditions. The pentameric form presents as an asymmetric structure, resembling the hexamer, but with the J-chain in the gap as opposed to the sixth IgM monomer (Hughey et al. 1998).
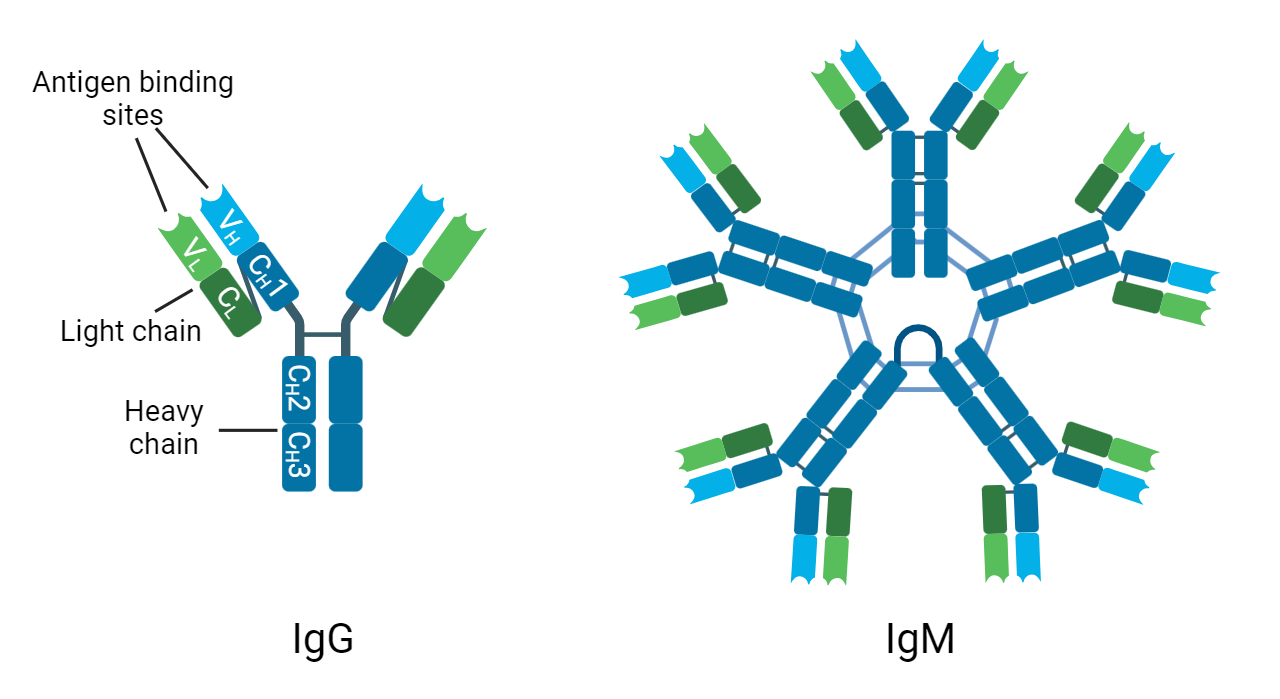
Fig. 1. The structure of IgG and IgM. C, constant region; H, heavy chain; L, light chain; V, variable region.
The structure of IgM has been shown to be important for its functions. For example, IgM is known to be a potent activator of the classical pathway of the complement system. Complement activation triggers a catalytic cascade, ultimately producing the membrane attack complex (MAC), which creates pores in the cell membrane of pathogens to provoke their death by osmolysis. Complement is initiated when the Fc regions of either IgG or IgM antibodies attached to the surface of a pathogen bind to a molecule known as C1q. However, C1q requires the binding of two Fc regions to become activated, meaning that while two separate molecules of IgG are necessary, the pentameric IgM structure is sufficient to incite rapid activation of the cascade (Janeway et al. 2001).
We also know that the J-chain plays an important role in mucosal immunity, as it allows secretory IgM to be translocated to the mucosal surfaces via the polymeric immunoglobulin receptor (pIgR), which is primarily located in the epithelial lining (Wei and Wang 2021).
The Present
So, we know the structure is important, but what’s new? Oskam et al. (2023) combined molecular biology and mass spectrometry (MS) techniques to specifically investigate the role of CD5 antigen-like (CD5L) protein, a member of the scavenger receptor cysteine-rich (SRCR) family, in IgM biology. It has previously been shown that CD5L — also known as apoptosis inhibitor of macrophage or AIM — can also bind in the gap of the murine pentameric IgM structure, attached via a disulfide bond (Hiramoto et al. 2018). But many aspects still remain a mystery. The precise functions of this protein and the significance of its binding to the IgM structure are largely unknown, not to mention the incidence of CD5L binding to IgM has varied widely in the literature.
Taking this into account, Oskam et al. performed size-exclusion chromatography (SEC) on pooled sera from 42 healthy donors and found over 99% of CD5L elution was in association with IgM and the J-chain. To confirm this, the researchers generated a monoclonal antibody that specifically recognizes the CD5L-IgM complex, allowing them to develop an ELISA to measure bound CD5L. Again, they observed a near-perfect correlation between CD5L-IgM complexes and total IgM content, reinforcing the idea that the CD5L-bound IgM complex is the predominant form in the circulation. Interestingly, when they examined secretory IgM, found in the saliva and breast milk, they noticed that it was mostly devoid of CD5L association.
Furthermore, after culturing naïve or memory B cells in vitro and analyzing the composition of the IgM secreted in the supernatant, the researchers realized that CD5L was not produced by the B cells themselves, rather B cell-derived IgM forms a complex with non-B cell-derived CD5L in the extracellular space.
Oskam et al. also assessed the function of the CD5L-bound IgM versus IgM alone, and determined that complement activation was unchanged, with both IgM forms equally stimulating the cascade. By utilizing ELISAs to look at the interaction of the bound and unbound IgM versions with the IgM Fc receptor (FcR) proteins, they found that binding to pIgR and FcαμR, which mediate mucosal transport and uptake of immune complexes by antigen-presenting cells, respectively, was reduced by the CD5L-bound form of IgM. Conversely, binding to FcμR, the receptor involved in regulating lymphocyte responses, was unchanged by the presence of CD5L. Taken together, these results indicate the association of CD5L with IgM can alter its functional activity.
The Future
Despite this development in the knowledge of the IgM structure, there is still a lot of research that needs to be done to fully understand the implications of CD5L association, and previous studies may need to be rethought to take into account CD5L. Oskam et al. speculated that CD5L-bound IgM has likely been overlooked until now due to the fact it is relatively small, taking up only around 4% of the total mass of IgM, and that it is assembled extracellularly, meaning that antibody studies using only immortalized B cell hybridomas will not produce CD5L. Moreover, the tools to measure CD5L-bound IgM were lacking before this study, which could also account for the underestimation of its significance. Therefore, the methods developed by Oskam et al. could be used to aid in future research.
Additionally, although typically monopolized by the IgG class, IgM is beginning to be regarded as a potential alternative format for the development of therapeutic antibodies (Zhang et al. 2022). Therefore, CD5L association may be an important aspect that needs to be contemplated and investigated for optimal efficacy of IgM as a biotherapeutic.
Overall, it appears that this old dog may have some new tricks after all.
Has This Study Piqued Your Interest in IgM?
Take a look at Bio-Rad’s range of antibodies against IgM for a wide range of target species.
References
Blandino R and Baumgarth N. (2020). Secreted IgM: new tricks for an old molecule. J Leukoc Biol 106, 1,021-1,034.
Hiramoto E et al. (2018). The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Sci Adv 4, eaau1199.
Hughey CT et al. (1998). Production of IgM hexamers by normal and autoimmune B cells: implications for the physiologic role of hexameric IgM. J Immunol 161, 4,091-4,097.
Janeway CA Jr et al. (2001). The distribution and functions of immunoglobulin isotypes. In Immunobiology: the immune system in health and disease. 5th edition. (New York, Garland Science).
Oskam N et al. (2023). CD5L is a canonical component of circulatory IgM. Proc Natl Acad Sci USA 120, e2311265120.
Schroeder HW and Cavacini L. (2013). Structure and function of immunoglobulins. J Allergy Clin Immunol 125, S41-52.
Wei H and Wang JY. (2021). Role of polymeric immunoglobulin receptor in IgA and IgM transcytosis. Int J Mol Sci 22, 2,284.
Zhang J et al. (2022). Therapeutic antibodies for COVID-19: is a new age of IgM, IgA and bispecific antibodies coming? MAbs 14, 2031483.
You may also be interested in...

View more Immunology or Article blogs















