Popular topics

-
References
Takizawa F et al. (1993). Binding of phycoerythrin and its conjugates to murine low affinity receptors for immunoglobulin G. Immunol Methods 18;162(2):269-72.
To control or not to control, that is the multi-color flow question…

Contemplating whether to use an isotype control in multi-color Flow Cytometry experiments could be a scene out of Shakespeare’s Hamlet! The topic appears to be one that divides researchers.
In this blog post, we provide you with an overview of what an isotype control is and how using one could be beneficial. We also describe the reasons why some scientists believe they are unnecessary. We’d like to hear your views on the “to control or not to control” debate so please use the feedback form below to leave a comment.
What is an isotype control and why is it used?
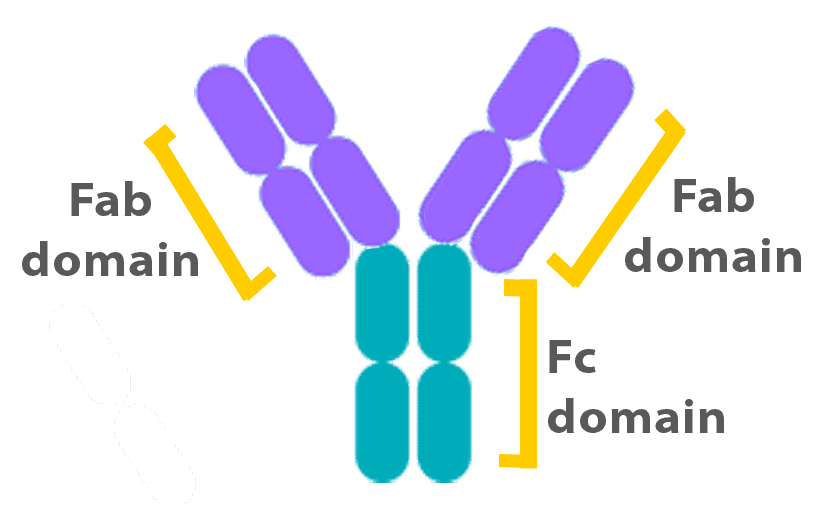
Antibodies consist of two ‘domains’, known as Fab and Fc (see Figure). While the Fab regions specifically bind to antigens, the Fc region binds to Fc receptors. Fc binding is essential for the activation of cells, such as macrophages, which express the receptor. In Flow Cytometry experiments, however, this type of binding can lead to false positive staining that originates from the antibody binding to Fc receptors rather than its target protein.
An isotype control is an antibody raised against an antigen not present on the cell type being analyzed. It is used in experiments to:
- Ensure that the observed staining is due to specific antibody binding to the target protein through the Fab domain
- Exclude non-specific binding of the antibody to Fc receptors
- Exclude other non-specific binding of the antibody or fluorophores to cellular components
To reduce the risk of Fc-mediated staining we recommend blocking Fc receptors with specialist blocking reagents, such as Bio-Rad's Mouse Seroblock reagent (BUF041), prior to adding the antibody. This step is particularly important when staining cell types that express many Fc receptors.
Get the most out of isotype controls
Using isotype controls for cell surface stainings
Isotype controls are recommended for cell surface stainings to monitor non-specific background staining. Here are our suggestions for controlling/including controls in your experimental design:
- Pick an isotype control that matches the host species, isotype and fluorophore of the primary antibody. If you are using a mouse IgG1 monoclonal antibody that is conjugated to FITC, you should therefore select a mouse IgG1 isotype control conjugated to FITC
- Accounting for non-specific fluorophore binding is especially important as certain fluorophores such as R-phycoerythrin (PE) have been shown to bind to certain types of mouse Fc-gamma receptors (Takizawa et al. 1993)
- As conjugation procedures and degree of antibody labeling (also known as F/P ratio; the ratio of fluorophore to an antibody) vary, it is best to purchase the isotype control from the same supplier to ensure you are comparing like for like
- Isotype controls should be used at the same concentration/dilution as the primary antibody
Using isotype controls for intracellular staining
Isotype controls are designed primarily for use in cell surface stainings. For intracellular staining experiments, there are special considerations especially when using FITC conjugates. Care should be taken as FITC has been shown to bind to intracellular components. When designing intracellular panels, it is therefore best to avoid FITC conjugates (Wingender 2013).
Typically, isotype controls are only one of several controls that should be performed in intracellular staining experiments. Other crucial controls include stimulation/blocking experiments and unstained cells (i.e. negative controls).
Why are scientists’ opinions divided about the value of isotype controls?
Isotype controls are mainly used to determine if the observed primary antibody binding is specific/real. They should be performed alongside other controls such as unstained cells (negative controls), compensation controls and Fluorescence Minus One (FMO) controls. FMO controls assist with identifying gating boundaries and some scientists feel that these are sufficient to negate the need for isotype controls. Others argue that FMO controls are not alternatives but additional controls to be used alongside isotype controls to fully demonstrate the validity of results.

Found our article useful? You may be interested in the following too...
Other controls
Other Flow posts
References
Takizawa F et al. (1993). Binding of phycoerythrin and its conjugates to murine low affinity receptors for immunoglobulin G. Immunol Methods 18;162(2):269-72.
You may also be interested in...

View more Applications or Flow Cytometry blogs