Antibodies for the Analysis of Kinases

- On This Page
- Antibodies for the analysis of kinases
- Antibodies for kinase research
- Kinase research antibody range
- References
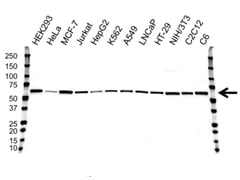
Western blot analysis of whole cell lysates probed with AKT1 antibody followed by detection with HRP conjugated Goat Anti-Mouse IgG (1/10,000, STAR207P) and visualized on the ChemiDoc MP with 19 second exposure. Arrow points to AKT1 (molecular weight 60 kDa).
Overview
During a phosphorylation event, a phosphate group is added by a kinase to a serine, threonine or tyrosine residue of a target protein. This process can be reversed by another group of enzymes called phosphatases.
Both kinases and phosphatases are specific with regards to whether they phosphorylate or dephosphorylate tyrosine or serine/threonine residues. Philip Cohen stated that “the reversible phosphorylation of proteins regulates nearly every aspect of cell life” (Cohen 2002). 518 kinases are encoded in the human genome (Manning et al. 2002; Ferguson and Gray 2018), which play a pivotal role in regulating cellular processes (Walsh et al. 2005; Ubersax and Ferrell 2007).
Protein kinases have developed a series of mechanisms to ensure specificity, for example structurally characteristic active sites and binding partners that ensure the kinase only phosphorylates its specific substrates (Ubersax and Ferrell 2007).
The process is also critical as aberrant phosphorylation levels and misregulation of phosphorylation cascades have been implicated in a variety of diseases, including cancer. Due to this correlation, much focus has recently been placed on developing specific kinase inhibitors as potential cancer treatments. One of these inhibitors currently used for the treatment of colorectal, head, and neck cancers is Cetuximab, a monoclonal antibody, which inhibits EGF receptors.
Antibodies for Kinase Research
Bio-Rad offers a variety of antibodies for analyzing kinases by ELISA, flow cytometry, immunofluorescence, immunohistochemistry and western blotting. For your convenience, we have grouped our research reagents into the seven main kinase families:
- AGC – there are 60 members of AGC kinase family, including PKA, PKC and PKG (Martin et al. 2008), which share a conserved catalytic kinase domain (Pearce et al. 2010). This family contains many core intracellular signaling kinases
- CAMK – includes calmodulin/calcium regulated kinases (Martin et al. 2008). These family members play key roles in neuronal transmission, synaptic plasticity, circuit development and cognition (Takemoto-Kimura et al. 2017)
- CK1 – small group of kinases that play regulatory roles in cell cycle, transcription, translation, cell-cell adhesion and receptor-coupled signal transduction (Schittek and Sinnberg 2014)
- CMGC – this conserved group consists of 62 members, including MAPK, CDK and GSK3 kinases (Martin et al. 2008 and Varjosalo et al. 2013)
- STE – many of the STE kinases are involved in MAPK kinase cascade signaling, transducing signals from the surface of the cell to the nucleus (Martin et al. 2008)
- TK - family members include receptor tyrosine kinases such as the EGF receptor (Martin et al. 2008)
- TKL – tyrosine like kinases are serine/threonine kinases that have a close sequence similarity to tyrosine kinases; family members include mixed lineage kinases (Martin et al. 2008)
- Other kinases
AGC |
CAMK |
CMGC |
STE |
TK |
TKL |
CK1 |
Other |
||||
AKT1AKT2PDK1Protein kinase CROCK1ROCK2SGK3 |
AMPK1AMPK2CAMKChk1Chk2Zip |
Cdk2Cdk4Cdk5Cdk6Cdk7Cdk9Erk1GSK3Jnk / MAPK8Jnk2 / MAPK9Jnk3 / MAPK10p38 / MAPK |
ASK1MAPKMEK1MEK2MEK3MEK4MEK5PAK1PAK2PAK4PAK6 |
Abl2AXLCD167CD246 / ALKc-MetCSKc-SrcEGF RErbB2 (HER2/neu)ErbB3ErbB4FAKFynLCKLynPKT7SykZap70 |
ALK1ILK1IRAK1IRAK2IRAK3IRAK4LIMK1LIMK2M3K11RAF1RIP1RIP3VEGFR1VEGFR2VEGFR3 |
CK1 |
Aurora A kinaseAurora B kinaseIKKNemo like kinasePINKPLK3ULK2WEE1 |
||||
Kinase Research Antibody Range
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|
- Growth factors
- EGF R resources
- mTOR signaling pathway
- Transcription factors
- Translation factors
- Phosphatases
- Phosphatases in cancer
- Phospho-Specific precisionAb antibodies
- Phospho-specific antibodies
- Sumoylation mediated signaling
- Ubiquitination mediated signaling
- Tyrosine phosphorylation of EGF R
- Signal transduction antibodies
References
- Cohen P (2002). The origins of protein phosphorylation. Nat Cell Biol 4, E127-30.
- Ferguson FM and Gray NS (2018). Kinase inhibitors: the road ahead. Nat Rev Drug Discov 17, 353-377.
- Manning G et al. (2002). The protein kinase complement of the human genome. Science 298, 1912-34.
- Martin et al. (2008). Kinomer v. 1.0: a database of systematically classified eukaryotic protein kinases. Nucleic Acids Research, doi: 10.1093/nar/gkn834.
- Pearce LR et al. (2010). The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11, 9-22.
- Schittek B and Sinnberg T. (2014). Biological functions of casein kinase 1 isoforms and putative roles in tumorigenesis. Mol Cancer 13, 231.
- Takemoto-Kimura S et al. (2017). Calmodulin kinases: essential regulators in health and disease. J Neurochem 141, 808-818.
- Ubersax JA and Ferrell JE Jr. (2007). Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol 8, 530-41.
- Walsh CT et al. (2005). Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl 44, 7342-72.
- Varjosalo M et al. (2013). The protein interaction landscape of the human CMGC kinase group. Cell Rep 3, 1306-20.


