Secondary Antibodies to CH2 and CH3 Domains

- On This Page
- The potential of therapeutic antibodies
- The role of antibody fragments
- CH2 and CH3 domains
The Potential of Therapeutic Antibodies
Therapeutic antibodies are a major strategy in many different types of therapy, for instance the treatment of cancer, inflammation and infectious diseases. Antibodies can be used to neutralize pathogens and toxins, tag cells for complement or cell mediated lysis, and alter cytoplasmic cascades. One of the main reasons antibody therapy is so versatile is because antibodies are able to bind to specific target cells. However, potential problems with antibody therapy are immunogenicity, stability, tissue penetration and accessibility of the targeted binding site. The latter two being largely due to their physical size.
The Role of Antibody Fragments
Antibody engineering has enabled the development of antibody fragments that have the same targeted specificity, but a smaller size, resolving problems with tissue penetration and target access. However, compared to full size antibodies, these fragments have a significantly reduced half-life. To overcome this, antibody fragments containing immunoglobulin (Ig) G Fc constant heavy chain 2 (CH2) regions have been developed. The CH2 in Ig classes IgG, IgA and IgD and the constant heavy chain 3 (CH3) in Ig classes IgE and IgM, form the Fc region of an antibody. Please refer to the IgG antibody image below to visualize where these regions are located on an antibody. The CH2 and CH3 regions within the Fc region are critical for Ig effector functions to elicit an immune response. The stability conferred by the IgG Fc CH2 fragment is a result of the binding of the neonatal Fc receptor (FcRn) to CH2, contributing to the long half-life of the Ig. FcRns natural function is in the stabilization of IgG as it transitions through the placenta to the fetus, prolonging the half-life of cellular IgG antibodies after birth.
IgG Antibody
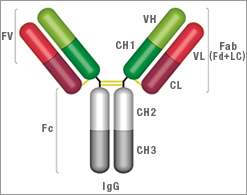 IgG Structure
IgG Structure
- Two heavy chains: each chain composed of VH, CH1, hinge region, CH2 and CH3
- Two light chains: each chain composed of VL and CL. There are two types of light chain, called kappa and lambda, always identical for each antibody
- Two antigen binding sites: found at the end of the VH and VL chain, known as the paratope
| Key | |
|---|---|
|
Fv = Fragment, variable region |
Fc = Fragment, crystallisable region |
|
VH = Variable heavy chain |
VL = Variable light chain |
|
CH1 = Constant heavy chain 1 |
CL = Constant light chain |
|
CH2 = Constant heavy chain 2 |
CH3 = Constant heavy chain 3 |
|
Fab = Fragment, antigen binding region |
Fd = Heavy chain of the Fab |
|
Lc = Light chain of the Fab |
Find out more about the IgG class and antibodies available to it by visiting our IgG Antibody page.
The development of antibody fragment libraries capable of binding multiple epitopes, has led to advances in antibody engineering and adaptation of the modular structure of antibodies, securing the future of antibody therapy.
The use of secondary antibodies to CH2 and CH3 domains enables Fc fragments to be studied, aiding the development of new therapeutic antibody fragments. Antibodies are available with a selection of chromogenic and fluorescent labels and suitable for many applications including ELISA, western blotting, immunohistochemistry, immunofluorescence and flow cytometry. Each is performance guaranteed for the application listed on the product datasheet.
- When using more than one secondary antibody ensure that they don’t cross react
- When working with some immune tissues or cells that contain a lot of Fc receptors, it helps to choose a F(ab) or F(ab’)2 fragment to eliminate non-specific binding. Alternatively, block Fc receptors via an absorption step, using purified IgG from the host species of your secondary antibody or serum from target cells
- View our IHC tips when using a secondary antibody
Any questions? Let us help, contact our technical support team for advice