Bovine Monocytes

- On This Page
- Overview
- Phenotype
- Immune function
- Antibodies and proteins
- Bovine monocytes antibodies
- References
Overview
Mammalian immune systems have evolved to defend the body against a large spectrum of microbial pathogens. A significant number of harmful agents are prevented from entering by the basic innate immune barriers of the mucosal and epithelial tissues. Pathogens that can defeat these initial defenses and infect the body will trigger the involvement of neutrophils, macrophages, and dendritic cells (DCs). An additional class of leukocytes, that has been found to play a larger role than expected, are monocytes.
Monocytes are circulating, mononuclear phagocytes relevant to both innate and adaptive immunity, with functions in immune defense, particularly in inflammation, and tissue remodeling. They are part of the mononuclear phagocyte system, along with embryonic macrophages and DCs. During inflammation, monocytes switch to differentiating into tissue macrophage and DC precursors. It has also been suggested that monocytes can present antigen to CD4+ and CD8+ T cells (Geissmann et al. 2010, Guilliams et al. 2014, and Jakubzick et al. 2017).
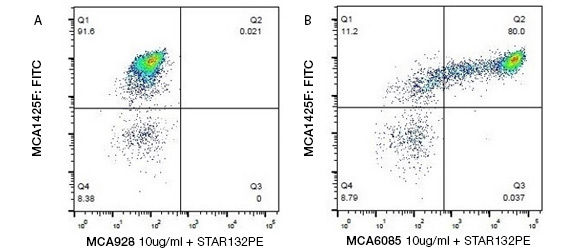
Staining bovine peripheral monocytes. Identifying bovine monocytes by two-color staining with Mouse Anti-Bovine CD14 Antibody, clone CAM36A (MCA6085), detected by Goat-Anti Mouse IgG1:PE Secondary Antibody (STAR132PE), and Mouse Anti-Bovine CD11b Antibody (MCA1425F).
Phenotype
Monocytes, like DCs, can be dived into subsets, with the discrimination based on the tasks the cells carry out in inflammation and tissue repair (Geissmann et al. 2003, Zawada et al. 2011). Three human monocyte populations have been discovered. These were differentiated on the basis of their CD14 and CD16 expression (Passlick et al. 1989, Ancuta et al. 2003). This has now been formulated as:
- The classical monocytes (cM) having the highest expression of CD14 but no CD16 (CD14++ CD16-)
- Intermediate monocytes (intM) with high CD14 and low CD16 expression (CD14++ CD16+)
- Non-classical monocytes (ncM) having low CD14 and high CD16 levels (CD14+ CD16++) (Ziegler-Heitbrock et al. 2010).
Similar to human monocytes, bovine monocytes can be subdivided into three groups:
- cM ─ high CD14 but no CD16 expression (CD14++ CD16−)
- intM ─ high CD14 and low CD16 expression (CD14++ CD16+)
- ncM ─ no CD14 but high CD16 expression (CD14− CD16++)
(Hussen et al. 2013, Corripio-Miyar et al. 2015, Elnaggar et al. 2016, Hussen et al. 2014).
The majority of bovine monocytes are of the cM subset (89%) with the remaining two, intM and ncM, each ranging from 5-10% for each subset. To detect all bovine monocytes, CD172a, also known as signal-regulatory protein alpha, can be used for analysis; although intM and ncM express higher levels (Hussen et al. 2013). Further markers to aid in subset differentiation are CD163, highly expressed on cM and MHC II, most abundantly found on intM.
Bovine cM also show the highest expression of L-selectin (CD62L) and Mac1 (CD11b/CD18); whereas intM have higher levels of the platelet endothelial cell adhesion molecule 1 (PECAM1, CD31) (Zawada et al. 2011). CD115, the pan human monocyte marker has only been confirmed by genomic data, as no antibody to bovine CD115 has been raised to date (Corripio-Miyar et al. 2015).
Immune Function
Key functions of monocytes, when defending against infection by pathogens, are phagocytosis and secretion of chemokines and cytokines. As may be expected, there are differences among the subsets in their abilities to carry out these functions. cM have the largest capacity for phagocytosis followed by the intM subset (Zawada et al. 2011). The latter is a higher producer of reactive oxygen species (ROS) and the inflammatory cytokines TNF-α and IL-1β. The CC chemokine (CCL)5 has so far been the only chemokine that is able to cause migration in bovine cM and induce differentiation into LPS responsive macrophages (Hussen J et al. 2014). While it is known that human monocytes are very responsive to CCL2 and bovine ones are not, further research needs to be conducted in this area.
Monocytes and neutrophils cooperate in innate immune responses during infection and inflammation. Neutrophil degranulation products (polymorphonuclear granulocytes degranulation products, PMN-DGP) are able to attract monocytes to inflammation loci and cause selective activation of bovine cM and intM in in vitro experiments (Hussen et al. 2016). They also induced high expression of CD11a and CD31 on intM.
Antibodies and Proteins
Bio-Rad is the leading supplier of species specific antibodies to veterinary and translational animal models. For over 25 years, Bio-Rad has offered the antibodies and relevant protein reagents needed for cutting edge bovine immune system research. Select from our bovine CD marker antibodies for immune cell analysis, and complement it with cytokine and chemokine ELISA pairs and protein standards for immune function analysis. For additional background information on the bovine immune system read our mini-review on bovine innate immunity.
Bovine Monocytes Antibodies
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|
References
- Ancuta P et al. (2003). Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 197(12), 1701–1707.
- Corripio-Miyar Y et al. (2015). Phenotypic and functional analysis of monocyte populations in cattle peripheral blood identifies a subset with high endocytic and allogeneic T-cell stimulatory capacity. Vet Res. 46, 112.
- Elnaggar M et al. (2016). Characterization and use of new monoclonal antibodies to CD11c, CD14, and CD163 to analyze the phenotypic complexity of ruminant monocyte subsets. Vet Immunol Immunopathol. 178, 57–63.
- Geissmann F et al. (2003). Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 19(1), 71–82.
- Geissmann F et al. (2010). Development of monocytes, macrophages, and dendritic cells. Science. 327(5966), 656–661.
- Guilliams M et al. (2014). Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 14(8), 571–578.
- Hussen J et al. (2013). Phenotypic and functional heterogeneity of bovine blood monocytes. PloS one. 8(8), e71502.
- Hussen J et al. (2014). The chemokine CCL5 induces selective migration of bovine classical monocytes and drives their differentiation into LPS-hyporesponsive macrophages in vitro. Dev Comp Immunol. 47(2), 169–177.
- Hussen J et al. (2016). Neutrophil degranulation differentially modulates phenotype and function of bovine monocyte subsets. Innate Immune. 22(2), 124–137.
- Jakubzick CV et al. (2017). Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 17(6), 349–362.
- Passlick B et al. (1989). Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 74(7), 2527–2534.
- Ziegler-Heitbrock L et al. (2010). Nomenclature of monocytes and dendritic cells in blood. Blood. 116 (16), e74–e80.
- Zawada AM et al. (2011). SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 118(12), e50–e61.


