Mini-review: Understanding Immuno-oncology
It is now appreciated that the immune system has the potential to eliminate cancer. A large number of immuno-oncology studies have shown that growing tumors can be recognized through a process referred to as cancer immunosurveillance. Clinically detectable tumors develop as a result of immunosuppressive mechanisms employed by the tumor. This understanding of the interplay between the immune system and cancer has led to the development of a number of immunological agents for cancer therapy. In this mini-review, we describe the field of immuno-oncology, focusing on the concept of cancer immunoediting and the most recent developments in cancer immunotherapy.
Introduction to immuno-oncology
One of the most recent developments in our understanding of cancer biology is the field of immuno-oncology. This area of cancer research involves the study of how the immune system interacts with a growing tumor. Despite the decades of cancer research dating back to the 18th century, it was not until the mid-20th century that the scientific community began to intensely investigate the role of the immune system in tumor development and progression (Parish 2003). Early studies on cancer focused on the cancer cell itself, with an emphasis on the genetic aspect of the disease. This was illustrated in the seminal, “Hallmarks of Cancer” article by Hanahan and Weinberg, published in 2000 (Hanahan and Weinberg 2000), which failed to acknowledge the role of the immune system in cancer development. A decade later in 2011, the article was updated to include “avoiding immune destruction” and “tumor-promoting inflammation” (Hanahan and Weinberg 2011) as hallmarks of cancer; indicating a new appreciation and further understanding of immuno-oncology.
The evidence forming the basis of immuno-oncology are studies showing that the immune system can recognize and reject tumors through a process described as cancer immunosurveillance (discussed in section 2). Clinical studies demonstrate that a subset of cancer patients have infiltrating T cells within the tumor, which correlate with a better prognosis. This has been observed in a wide range of cancers including breast cancer, renal cell carcinoma, melanoma, ovarian cancer and gastrointestinal stromal tumors (Gajewski et al. 2013).
Based on the logic that a growing tumor can evade or suppress host immune mechanisms, the immune system can be targeted to suppress the tumor. Accordingly, the main goals of cancer immunotherapy are to strengthen the patient’s immune response to the tumor by improving its capacity for tumor recognition and the disruption of immunosuppressive mechanisms (discussed in section 3).
Overview of the cancer immunoediting model
In 1908, Paul Ehrlich speculated that cancer could occur at “overwhelming frequency” if it was not for host immune responses preventing the outgrowth of continuously arising cancer cells (Ehrlich 1908). At the time, it was difficult to test this hypothesis experimentally as there was limited understanding of the immune system, and appropriate models such as inbred transgenic mice were not yet available. In the late 1950s, studies demonstrating the existence of tumor associated antigens led Burnet and Thomas to build on Ehrlich’s observations, thereby proposing the cancer immunosurveillance hypothesis (Burnet 1957, Thomas 1959). This hypothesis states that cellular immune mechanisms can recognize unique antigens expressed by cancer cells and eliminate them before they present clinically as tumors (Lussier and Schreiber 2016). These tumor antigens can be viral proteins found in tumors caused by viruses, neo-antigens from mutated proteins or over expressed self antigens.
The concept of cancer immunosurveillance was demonstrated through several studies using inbred genetically modified mice that lack relevant immune cell types such as T and B cells. For example, it has been shown that mice deficient in functional natural killer (NK) T cells, γδ T cells, NK cells, αβ T cells, interferon (IFN)-γ or interleukin (IL)-12 demonstrate increased susceptibility to tumors (Dunn et al. 2002) compared to wild type mice. Similarly, in clinical studies, immunosuppressed transplant patients and individuals with various immunodeficiencies show a significantly higher relative risk for cancer development (Penn 1970, Gatti and Good 1971).
Recently, the cancer immunosurveillance hypothesis has evolved into the “cancer immunoediting” model, which provides further details on the interaction between the immune system and cancer. In contrast to the immunosurveillance hypothesis, the immunoediting model emphasizes the ability of the immune system to not only eliminate highly immunogenic cancer cells, but to also produce cancer cell variants with reduced capacity to provoke an anti-tumor immune response (Dunn et al. 2006, Shankaran et al. 2001). Cancer immunoediting is further described as a process driven by the immunogenicity of tumor antigens, which results in either tumor rejection (elimination phase), immune control of the growth of residual cancer cells (equilibrium phase), or tumor-mediated evasion of immune control (escape phase) (Schreiber et al. 2011, Mittal et al. 2014) (Figure 1). Below we describe these three Es of cancer immunoediting.
Three stages of immunoediting
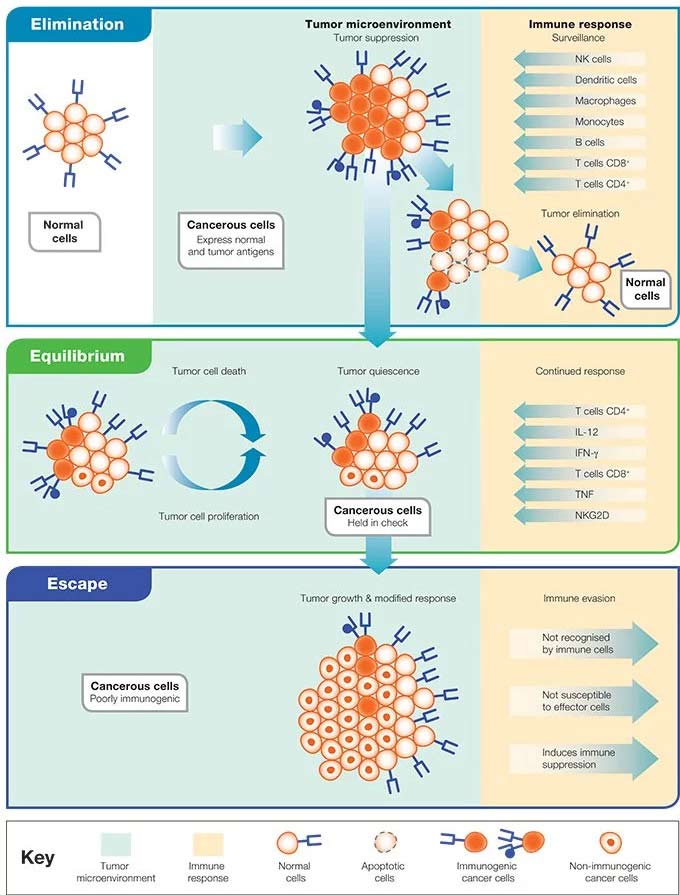
Figure 1. The three Es of cancer immunoediting. Adapted from Dunn et al. 2002.
Elimination phase — immune mechanisms of tumor clearance
Cancer immunosurveillance occurs during the elimination phase of cancer immunoediting. At this stage, both innate and adaptive immunity coordinate to scan the body for abnormal cells and destroy early tumors before they are clinically visible (Mittal et al. 2014). When a normal cell is transformed by factors such as carcinogen exposure or genetic mutations, it begins to express molecules that alert the immune system that it is foreign and should be removed. These include tumor antigens presented in the context of major histocompatibility class I (MHC I) molecules, the heat shock protein calreticulin as well as NKG2D ligands. These molecules are recognized by cytotoxic CD8+ T cells, dendritic cells (DCs) or NK cells, respectively.
The first few transformed cells are initially detected by NK cells through their encounter with specific ligands on tumor cells. NK cells induce apoptosis in cancer cells by producing cytotoxic molecules such as granzyme and perforin or through antibody-dependent cellular cytotoxicity (ADCC).
DCs or macrophages then engulf the dying tumor cells, and process and cross-present the tumor antigens to CD8+ T cells, CD4+ T cells, NKT cells or B cells. The activated effector T cells then secrete the pro-inflammatory cytokine IFN-γ, which is critical for inhibiting tumor proliferation and angiogenesis (Dunn et al. 2006). Specifically, activated B cells produce tumor specific antibodies that can mediate ADCC by NK cells as well as further prime CD4+ and CD8+ T cells.
Similar to NK cells, cytotoxic CD8+ T cells induce apoptosis in cancer cells through the secretion of perforin and granzymes, as well as interaction with Fas and TRAIL receptors on cancer cells. Effector T cells also express co-stimulatory molecules such as CD28, OX40 and CD137 that mediate their proliferation and survival. In addition, γδ T cells recognize and kill cancer cells expressing NKG2D ligands by apoptosis (Finn 2012). These cells can also present peptide antigens derived from dying cancer cells to α/β T cells (Wu et al. 2014).
The full power of the adaptive immune system leads to the elimination of remaining tumor cells and the generation of immunological memory to specific components of the tumor to prevent tumor recurrence (Finn 2012).
The table below shows the immunophenotype and roles of the main immune cells involved in tumor clearance during the elimination phase of cancer immunoediting.
Table 1. Immunophenotype and roles of major immune cell types involved in tumor clearance
Immune Cell |
Mouse Markers |
Human Markers |
Role In Tumor Clearance |
|---|---|---|---|
|
CD8+ cytotoxic T cells |
Recognize tumor antigens and initiate anti-tumor responses such as induction of apoptosis and secretion of pro-inflammatory cytokines such as IFN-γ |
||
|
CD4+ T cells |
Drive both the antibody response and the CD8+ cytotoxic T cell response. Contribute to inflammatory environment through secretion of inflammatory cytokines |
||
|
γδ T cells |
Recognize and induce apoptosis in cancer cells expressing NKG2D ligands. Secrete IFN-γ, and present peptide antigens from dying tumor cells to α/β T cells |
||
|
Natural killer cells |
CD56, CD57, CD94, CD159a, CD335, CD336, CD337, granzyme B |
Identify cancer cells using inhibitory receptors that scan for altered protein expression patterns on tumors. Initiate cell death via antibody dependent cellular cytotoxicity or perforin/granzyme B mediated apoptosis. Secretion of pro-inflammatory cytokines such as IFN-γ, TNF and IL-6 |
|
|
Activated B cells |
Secrete tumor reactive IgG antibodies. Promote tumor killing by NK cells, phagocytosis by macrophages and priming of CD4+ and CD8+ T cells |
||
|
Dendritic cells |
Respond to danger signals secreted by dying tumor cells; engulf and process tumor cells and present antigens in the context of MHC molecules to cognate T cells to initiate anti-tumor immune response. Provide co-stimulatory signal for maturation and differentiation of naïve T cells to effector T cells. Secrete pro-inflammatory cytokines such as IL-12 |
IFN, interferon; TNF, tumor necrosis factor; IgG, immunoglobulin G; NK cells, natural killer cells; IL, interleukin
Equilibrium phase - tumor dormancy
While cancer immunosurveillance mechanisms are powerful and can eliminate a significant percentage of transformed cells, some can escape immune control and survive.
In the equilibrium phase, the surviving cancer cells are held in a state of immune-mediated dormancy, in which cancer cells are eliminated at the same rate as cancer proliferation. There is also a balance in the production of anti-tumor cytokines (IL-12, IFN-γ) and tumor promoting cytokines (IL-10, IL-23) (Mittal et al. 2014). A study comparing the microenvironment of equilibrium phase tumors and those escaping immune control, demonstrated that high proportions of CD8+ T cells, NK cells and γδ T cells, and low numbers of IL-13 producing NKT cells, Foxp3+ T-regulatory cells (Tregs), and myeloid derived suppressor cells (MDSCs) are necessary to maintain the equilibrium phase (Wu et al. 2013).
During this phase, some cancer cells undergo an editing process featuring genetic and epigenetic changes due to the constant immune pressure. As a result, cancer cell variants emerge that possess the ability to evade immune recognition and induce immunosuppression (Mittal et al. 2014).
Escape phase - Mechanisms of immune suppression
Cancer cell variants that evolve during the equilibrium phase do so by employing a number of immunosuppressive mechanisms. At this stage, tumor growth proceeds without restriction from immune pressure and can now be detected clinically. The mechanisms used by the tumor to inhibit the protective functions of the immune system are often complementary and may act in parallel.
To evade immune recognition, cancer cells often downregulate their expression of tumor antigens, MHC class I or co-stimulatory molecules. Cancer variants may also increase their expression of resistance molecules such as STAT3, which suppresses the induction of immune stimulatory molecules such as calreticulin, leading to impaired maturation of DCs. To enhance their survival, cancer cells promote resistance to apoptosis through increased expression of anti-apoptotic molecules such as Bcl-2 (Mittal et al. 2014).
To suppress immune function, cancer cells may secrete paracrine mediators such as adenosine, and prostaglandin E2, as well as suppressive cytokines such as IL-10, TGF-beta, IL-6 and M-CSF. These mediators have multiple effects such as suppression of DCs, indirect inhibition of T cell penetration into the tumor bed, direct inactivation of effector T cells and activation of suppressive Tregs (Mellman et al. 2011). Other mechanisms include secretion of soluble NKG2D ligands, which severely inhibit γδ T cell mediated cytotoxicity (Groh et al. 2002).
Cancer cells can also upregulate surface ligands such as PD-L1 that block T cell function by inducing T cell anergy or exhaustion. T cells, including Tregs, can also express inhibitory receptors such as PD-1, CTLA-4, Tim-3 and LAG-3, which suppress anti-tumor immunity and promote tumor growth (Mittal et al. 2014). Cancer cells also overproduce other inhibitory molecules of T cell function such as indoleamine 2,3-dioxygenase, galectin-1/3/9, CD39 and CD73.
Moreover, the tumor can also recruit immune cell types that suppress immune function to the tumor bed, thereby promoting tumor growth. These suppressive immune cell types include IL-10 producing B cells, B-regulatory cells, MDSCs, IL-13 producing NKT cells, NK cells and γδ T cells. MDSCs in particular can suppress T and NK cell activation through the production of nitric oxide, reactive oxygen species, arginase, IL-10 and TGF-beta (Gabrilovich and Nagaraj 2009).
As the tumor continues to grow by employing the above mechanisms, other cell types in the tumor stroma can also mediate immune modulation. Cancer-associated fibroblasts can facilitate the recruitment and function of immunosuppressive cells by secreting the chemokines CCL2 and CXCL2. They also secrete TGF-beta to inhibit effector T cell function (Mellman et al. 2011). Myeloid derived mesenchymal stem cells within the tumor bed can also block the proliferation and effector functions of T cells (Mellman et al. 2011).
In the escape phase, cancer cell variants effectively bypass immune control. Accordingly, scientists are targeting these known mechanisms of immune evasion in order to develop effective cancer immunotherapy approaches. The ultimate goal is to skew the immune system towards tumor elimination rather than tumor progression. Table 2 summarizes the immunosuppressive mechanisms employed by cancer cells.
Table 2. Summary of cancer immunosuppressive mechanisms
Overall Tumor Escape Strategy |
Mechanisms Of Action |
|---|---|
|
Evasion of immune recognition |
Downregulation of MHC class I molecules Loss of tumor antigens Down-regulation of co-stimulatory molecules |
|
Resistance to immune effector mechanisms |
Secretion of NKG2D ligands to inhibit T cell mediated cytotoxicity Resistance to apoptosis induction (e.g. upregulation of Bcl-2) Suppression of danger signals via STAT3 upregulation |
|
Induction of immune suppression |
Secretion of paracrine mediators and suppressive cytokines such as adenosine, prostaglandin E2, IL-10, TGF-beta, IL-6 and M-CSF Upregulation of surface ligands that block T cell function or induce T cell anergy/exhaustion e.g. PD-L1 Overproduction of T cell inhibitory molecules such as IDO, galectin-1/3/9, CD39 and CD73 Recruitment and induction of immunosuppressive cells such as Tregs, MDSCs, cancer-associated fibroblasts and myeloid derived mesenchymal stem cells within the tumor bed |
Adapted from Mittal et al. 2014 and Mellman et al. 2011. Bcl-2, B cell lymphoma 2; IDO, indoleamine 2,3-dioxygenase; MDSCs, myeloid derived suppressor cells; MHC, major histocompatibility complex; M-CSF, macrophage colony-stimulating factor; PD-L1, programmed death-ligand 1; STAT 3, signal transducer and activator of transcription-3; TGF-beta, transforming growth factor-beta.
Advances in cancer immunotherapy
The goal of cancer immunotherapy is to boost the body’s own response against cancer, and block any mechanisms that prevent effective anti-tumor immunity. Considering the various immunosuppressive strategies employed by cancer (Table 2), the success of immunotherapy may seem unlikely. However, there have been several recent advances in cancer immunotherapy, some of which have demonstrated clinical success.
The strategies currently being explored for cancer immunotherapy center on either stimulating immune effector mechanisms or counteracting suppressive mechanisms. The approaches include the use of cancer vaccines, adoptive T cell therapy, oncolytic viruses and inhibitory antibodies. Below, we summarize these approaches and highlight successful therapeutics.
Cancer vaccines
While preventative cancer vaccines have shown considerable success with cancers of viral origin such as hepatitis B virus and human papillomavirus (HPV), therapeutic cancer vaccines have struggled to achieve the same success (Mellman et al. 2011). The knowledge that cancer patients often possess CD8+ and CD4+ T cells that can respond to tumor antigens was the catalyst for the development of therapeutic cancer vaccines. Therefore, the main purpose of cancer vaccines is to supply immunogenic tumor antigens that can further stimulate effector T cells and drive anti-tumor immunity (Mellman et al. 2011). However, it is now appreciated that in order to be effective, cancer vaccines must overcome the immune tolerance acquired by tumor cells. This requires the targeting of large quantities of tumor antigens to DCs, which must then in turn be expanded and activated using appropriate agents (Yaddanapudi et al. 2013).
Cancer vaccine formulations currently being explored include crude tumor lysates, purified tumor antigens, whole tumor cells, tumor cells genetically engineered to express immunostimulatory cytokines, as well as DNA/RNA molecules encoding various tumor antigens (Yaddanapudi et al. 2013). Most of these approaches have failed in clinical trials largely due to a paucity of universal antigens as well as poor immunization protocols, leading to poor efficacy and clinical response (Farkona et al. 2016).
The Sipuleucel-T vaccine for prostate cancer has been the most successful therapeutic cancer vaccine to date, and was approved by the United States Food and Drug Administration (FDA) in 2010. It is developed from enriched blood antigen presenting cells (APCs) isolated from the patient. The APCs are then cultured with a fusion protein consisting of prostatic acid phosphatase (PAP) linked to granulocyte macrophage colony-stimulating factor (GM-CSF), a DC growth and differentiation factor. This vaccine stimulates T cell responses against PAP, which is expressed on prostate cancer cells but not on non-prostate tissues. Sipuleucel-T can induce a 4 month improvement in median survival (Kantoff et al. 2010). However, a major limitation of the vaccine is that this enhanced patient survival is not accompanied by tumor shrinkage (Kantoff et al. 2010).
Current studies on the development of therapeutic cancer vaccines are focusing on improving their efficacy by overcoming the immunosuppression in cancer. Other areas of focus include exploration of predictive biomarkers for diagnostic identification of patients most likely to benefit from a specific vaccine based on the tumor antigens they express (Mellman et al. 2011, Farkona et al. 2016).
Adoptive T cell therapy
Adoptive T cell therapy (ACT) harnesses the ability of effector T cells to recognize and eliminate cancer cells. T cells are first isolated from a patient’s blood, tumor tissue or tumor draining lymph node, manipulated and expanded ex vivo, and then reinfused into the patient (Hinrichs and Rosenberg 2014). This approach circumvents the issue of breaking tolerance to tumor antigens and produces a significant amount of high-avidity effector T cells (Farkona et al. 2016).
The adoptive transfer of ex vivo expanded tumor infiltrating lymphocytes (TILs) combined with lymphodepletion led to complete and durable clinical responses in a subset of patients with metastatic melanoma (Dudley et al. 2002). Other clinical trials using ACT with TILs combined with lymphodepletion have reported consistently high response rates and long-lasting tumor regression (Yee 2013).
Despite the success of this immunotherapeutic approach, there are some disadvantages. For example, lymphodepletion can be life threatening, particularly when ablative radiation therapy is used. In addition, while TILs can be isolated from many cancers, only those from melanoma have demonstrated clinical success (Farkona et al. 2016).
To address these limitations, advances in T cell culturing and engineering has led to new ACT approaches. The strategies currently being explored in the clinic include the use of retroviral vectors harboring cloned T cell receptors (TCRs) or chimeric antigen receptors (CARs). The first approach utilizes TCRs with α and β chains that provide the engineered T cell with the antigen specificity of the TCR. This therapy will be useful for patients whose tumors express the specific antigen for which the TCR was engineered. However, there have been detrimental side effects from the destruction of healthy tissues that also express the target antigen.
The second approach involves CARs, which consist of an Ig variable domain linked to a TCR constant domain. Engineered CAR T cells have the antigen-recognition properties of antibodies, thus bypassing the need for antigen processing and presentation. They can therefore be targeted to any antigen expressed on the tumor cell surface (Farkona et al. 2016).
The use of these genetically engineered T cells has led to observed tumor regression in B cell malignancies, melanoma and synovial sarcoma (Hinrichs and Rosenberg 2014). Clinical trials utilizing these T cells are also underway for other types of cancers (Mellman et al. 2011). Future studies aim to address the lack of appropriate target antigens for effective ACT as well as safety issues surrounding the selection of the target (Mellman et al. 2011).
Oncolytic virus therapy
Immunotherapy of cancers using oncolytic viruses is a novel approach that utilizes native or engineered viruses to selectively replicate in and kill cancer cells (Lichty et al. 2014). The two main mechanisms of action of oncolytic viruses include debulking of the tumor through cancer cell infection, as well as cell lysis and subsequent induction of systemic anti-tumor immunity (Lichty et al. 2014). Accordingly, one major advantage of this immunotherapeutic approach is that it induces minimal toxicity to normal tissues.
In addition to the limited toxicity, oncolytic viruses can also be engineered to express specific cytokines that favor immune cell recruitment and activation (Farkona et al. 2016). One such successful oncolytic therapy is Talimogene laherparepvec (T-VEC), which was approved by the FDA for the treatment of advanced melanoma in October 2015 (Ledford 2015).
T-VEC is a modified oncolytic herpes simplex virus type 1 with genes deleted to prevent neuronal involvement. These deleted genes were replaced with GM-CSF, which induces DC recruitment to the tumor microenvironment and increases oncolytic therapeutic activity (Ledford 2015).
Despite the success of T-VEC, there are still some noted limitations to oncolytic virotherapy that need to be addressed before other such therapies can be brought to clinic. For instance, although T-VEC demonstrates low toxicity, it is less efficient in patients with more advanced disease (Farkona et al. 2016).
In addition, because oncolytic viruses are injected into the tumor to avoid host antiviral immunity, this approach may not be suitable for tumors in organs that are difficult to reach with an injection or for treating disseminated metastatic tumors. However, the success of T-VEC encourages further study into the development of oncolytic viruses, and this continues to be an active area of research.
Immune checkpoint inhibitor antibodies
The immune system consists of a number of mechanisms that put the brakes on the immune response in order to avoid hyper activation and autoimmunity. These mechanisms are referred to as immune checkpoints, and the term “checkpoint blockade” describes the blocking of these immune suppressive mechanisms activated by cancer (Farkona et al. 2016). One immunotherapeutic approach has therefore been to develop agents that specifically target key molecules in immune inhibitor pathways, consequently taking the brakes off the immune system and promoting anti-tumor immune function.
The first checkpoint inhibitor drug approved by the FDA in 2011 as a first-line therapy for metastatic melanoma was ipilimumab, a monoclonal anti-CTLA-4 antibody (Hodi et al. 2010). CTLA-4 is an inhibitory receptor that downregulates the early stages of T cell activation. Ipilimumab has demonstrated significant clinical benefit, with prolongation of overall survival observed in a significant number of patients (Hodi et al. 2010). However, some limitations to anti-CTLA-4 therapy include severe adverse immune-related events as well as a delayed therapeutic response since the clinical benefit of ipilimumab can take several months to manifest (Hodi et al. 2010, Mellman et al. 2011).
Since the approval of ipilumimab, other immune checkpoint inhibitor antibodies have emerged as viable options for treating a number of cancers with a focus on inducing less severe side effects. These antibodies specifically target the PD1/PD-L1(2) axis. PD1 is another inhibitory receptor expressed on antigen-stimulated T cells. Its activation inhibits T cell proliferation, cytokine release and cytotoxicity (Farkona et al. 2016). The ligands for PD1 are PD-L1 and PD-L2, which are expressed within the tumor. In the escape phase of cancer development, PD1/PD-L1(2) signaling is activated, leading to inactivation of the anti-tumor immune response.
The anti-PD1 antibody, nivolumab, has demonstrated long lasting clinical responses in advanced melanoma, with some patients remaining free from disease progression for many years (Topalian et al. 2014). The anti-PD-L1 antibody atezolizumab demonstrated significant therapeutic responses across a wide range of cancers including lung, colon, head and neck, renal cell carcinoma, melanoma and gastric cancers (Farkona et al. 2016). The anti-PD1 antibodies pembrolizumab and nivolumab have been FDA approved for treating melanoma and non-small cell lung cancer, with nivolumab also approved for treatment of renal cell carcinoma (Sharma and Allison 2015).
In contrast to ipilumimab, antibodies targeting the PD1-PD-L1 axis demonstrate a favorable toxicity profile, with the majority of reported cases of toxicity being readily manageable with supportive care (Brahmer et al. 2012).
Multiple other immune checkpoint pathway molecules have been targeted for cancer therapy and are being evaluated in preclinical tumor models or clinical trials (Farkona et al. 2016). These include the lymphocyte activation gene 3 (LAG3) protein and T cell immunoglobulin and mucin domain-containing 3 (TIM3) protein.
LAG3 is expressed on exhausted T cells and is a selective marker of Tregs, suggesting that it may play a role in immune suppression. Therapeutics targeting LAG3 are being tested in clinical trials either as a monotherapy or in combination with anti-PD1 antibodies, with encouraging results (Sharma and Allison 2015).
A number of T cell subsets as well as DCs express TIM3. It is also expressed with PD1 on CD8+ T cells. Therefore combination therapies targeting both PD1 and TIM3 are currently being tested in preclinical tumor models (Farkona et al. 2016). In addition to LAG3 and TIM3, other immune checkpoint inhibitor agents are also being evaluated (Mellman et al. 2011).
Our current understanding of how the immune system interacts with cancer has revolutionized the approach to cancer treatment. Recent studies indicate that well designed immunotherapies, administered at the appropriate stage of tumor progression can tilt the on-going interaction of the tumor with the immune system from immune escape to cancer elimination.
References
- Brahmer JR et al. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366, 2455-2465.
- Burnet FM (1957). Cancer – a biological approach. I. The processes of control. Br Med J 1, 779-786.
- Dudley ME et al. (2002). Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850-854.
- Dunn GP et al. (2002). Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3, 991-998.
- Dunn GP et al. (2006). Interferons, immunity and cancer immunoediting. Nat Rev Immunol 6, 836-848.
- Ehrlich P (1908). Üeber den jetzigen stand der Karzinomforschung. Ned Tijdschr Geneeskd 5, 273-290.
- Farkona S et al. (2016). Cancer immunotherapy: the beginning of the end of cancer? BMC Medicine 14, 73.
- Finn OJ (2012). Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 23, viii6-viii9.
- Gabrilovich DI and Nagaraj S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9, 162-174.
- Gajewski TF et al. (2013). Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14, 1014-1022.
- Gatti RA and Good RA (1971). Occurrence of malignancy in immunodeficiency diseases. A literature review. Cancer 28, 89-98.
- Groh V et al. (2002). Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419, 734-738.
- Hanahan D and Weinberg RA (2000). The hallmarks of cancer. Cell 100, 57-70.
- Hanahan D and Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell 144, 646-674.
- Hinrichs CS and Rosenberg SA (2014). Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev 257, 56-71.
- Hodi FS et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363, 711-723.
- Kantoff PW et al. (2010). Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 363, 411-422.
- Ledford H. (2015). Cancer-fighting viruses win approval. Nature 526, 622-623.
- Lichty BD et al. (2014). Going viral with cancer immunotherapy. Nat Rev Cancer 14, 559-567.
- Lussier DM and Schreiber RD (2016). Cancer immunosurveillance: immunoediting. Reference module in biomedical sciences. 4, 396-405.
- Mellman I et al. (2011). Cancer immunotherapy comes of age. Nature 480, 480-489.
- Mittal D et al. (2014). New insights into cancer immunoediting and its three component phases- elimination, equilibrium and escape. Curr Opin Immunol 27, 16-25.
- Parish CR (2003). Cancer immunotherapy: The past, the present and the future. Immunol Cell Biol 81, 106-13.
- Penn I (1970). Malignant tumors in organ transplant recipients (New York: Springer-Verlag).
- Schreiber RD et al. (2011). Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565-1570.
- Shankaran V et al. (2001). IFN gamma and lymphocytes prevent primary tumor development and shape tumour immunogenicity. Nature 410, 1107-1111.
- Sharma P and Allison JP (2015). Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205-214.
- Thomas L (1959). In Cellular and humoral aspects of the hypersensitive states. Lawrence HS ed. (Hoeber-Harper: New York), pp 529-532.
- Topalian SL et al. (2014). Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32, 1020-1030.
- Wu X et al. (2013). Immune microenvironment profiles of tumor immune equilibrium and immune escape states of mouse sarcoma. Cancer Lett 340, 124-133.
- Wu YL et al. (2014). γδ T cells and their potential for immunotherapy. Int J Biol Sci 10, 119-135.
- Yaddanapudi K et al. (2013). Cancer vaccines. Oncoimmunology 2, e23403.
- Yee C (2013). Adoptive T-cell therapy for cancer: boutique therapy or treatment modality? Clin Cancer Res 19, 4550-4552.







