Staining of intracellular antigens requires fixation followed by permeabilization, allowing antibodies to reach their target. These techniques, along with resources and products to optimize your staining, are described here.
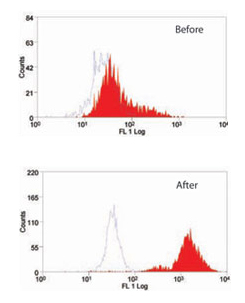
Fig.1. The effect of Leucoperm on (MCA519G) which recognizes MOMA-2, an intracellular antigen in mouse macrophages and monocytes.
Products
Leucoperm
Leucoperm™ makes intracellular antigen analysis using flow cytometry easy. The kit comes with a ‘Reagent A’ that fixes cells in suspension, and a ‘Reagent B’ that subsequently permeabilizes them.
- Results within the hour
- Negligible background staining
- Optimal staining patterns
- Excellent scatter characteristics
With Leucoperm, antibodies can access intracellular structures leaving the morphological scatter characteristics of the cells intact. Leucoperm is superb for detecting many intracellular antigens including:
- Cytokines e.g. IL-2 and IFN
- TdT, MPO, and CD3 involved in leukemia and lymphoma
- Platelets: P-selectin, CD63, and thrombospondin
- Nuclear Ki-67 and PCNA expression in proliferating cells
The Ideal Fixation and Permeabilization Kits
The specific formulation of Leucoperm reduces background staining and allows the simultaneous addition of permeabilization medium and fluorochrome-labeled antibodies.
Most commercially available monoclonal antibody conjugates can be used with Leucoperm reagents.
Erythrolyse Red Blood Cell Lysing Buffer
Bio-Rad Erythrolyse is designed for use in whole blood immunofluorescent staining procedures and is suitable for use with human, rat, and mouse blood.
Protocol
Direct Staining of Intracellular Antigens: Leucoperm Accessory Reagent Method

Direct staining of intracellular antigens and cytokines by flow cytometry: Leucoperm method
Method for cell permeabilization required prior to intracellular staining using Leucoperm Accessory Reagent.
The detection of intracellular antigens requires a cell permeabilization step prior to staining. For the detection of cell cycle antigens such as PCNA and BrdU, methanol modification is recommended — see protocol FC8 (Direct staining of intracellular antigens by Flow Cytometry: Leucoperm plus methanol method).
The method described below produces excellent results in our hands; however, other permeabilization techniques have been published, and may also be used successfully for this application. These methods should always be used in conjunction with the product and batch specific information provided with each vial. A certain level of technical skill and immunological knowledge is required for the successful design and implementation of these techniques; these are guidelines only and may need to be adjusted for particular applications. Note: Specific methodology for blood appears in [ ] brackets.
Reagents
- Leucoperm Accessory Reagent (Product code BUF09). Includes Reagent A (cell fixation agent) and Reagent B (cell permeabilization agent).
- Phosphate buffered saline (Product code BUF036A) containing 1% bovine serum albumin (PBS/BSA).
- Erythrolyse red blood cell lysing buffer (BUF04).
- Anticoagulant (Note: for basic staining any appropriate anticoagulant, such as heparin, EDTA, or acid citrate dextrose, may be used. In some instances specific anticoagulants may be required)
- Optional: 0.5% (w/v) paraformaldehyde in PBS (Note: dissolve on heated stirrer and cool before use)
Method
-
Harvest cells after appropriate treatment and determine the total number present. Adjust cell suspension to a concentration of 1 x 107 cells/ml with cold (4oC) PBS/BSA.
[Whole blood samples may be used undiluted unless the cell count is high, as in leukemia samples. Use appropriate anticoagulant]
- Add 100 μl of cell suspension [or whole blood] to the appropriate number of test tubes.
- If required, perform staining of cell surface antigens using appropriate directly conjugated monoclonal antibodies at 4oC, avoiding direct light.
- Wash cells once in 2 ml cold (4oC) PBS/BSA and discard the supernatant.
- Re-suspend cells in 100 ul cold (2-8oC) Leucoperm Reagent A (cell fixation agent) per 1 x 106 cells. Incubate for 10 min at 2-8oC.
- Add 3 ml room temperature PBS/BSA and centrifuge for 5 min at 300-400 g at room temperature.
-
Remove supernatant and add 100 μl Leucoperm Reagent B (cell permeabilization agent) per 1 x 106 cells. Add the directly conjugated antibody at the vendor-recommended dilution and incubate at 4oC for at least 30 min, avoiding direct light.
[To the blood suspension add 2 ml freshly prepared erythrolyse red cell lysing buffer and mix well. Incubate for 10 minutes at room temperature. Centrifuge at 300-400 g for 5 minutes and discard the supernatant. Wash with 2 ml room temperature PBS/BSA, centrifuge at 300-400g for 5 minutes at room temperature and discard the supernatant. Continue to step 8.]
- Wash once in PBS and then re-suspend in 200 μl cold (4oC) PBS for immediate analysis or with 200 μl of 0.5% formaldehyde in PBS if required.
- Acquire data by flow cytometry. Analyze fixed cells within 24 hours.
Notes
-
The following should be considered when designing your flow cytometry experiments:
-
To avoid nonspecific binding, you also need to block Fc receptors on cell types such as spleen cells, with FcR blocking reagents e.g. Bio-Rad’s Mouse Seroblock reagent (BUF041).
-
Appropriate controls should always be carried out, for flow cytometry the following should be considered for inclusion;
-
Isotype controls used to determine if the staining is specific.
-
Unstained cells should always be included in the experimental set-up to monitor autofluorescence.
-
- For all multicolor flow cytometry experiments it is advisable to include compensation controls and Fluorescence Minus One (FMO) controls, which assist with identifying gating boundaries.
-

Tips and Tricks for Intracellular Flow Cytometry
Resources
Intracellular Flow Cytometry
Flow cytometry protocols and staining procedures vary depending on whether the antigen to be detected is located on the cell surface or intracellular. Detection of cell surface proteins, such as CD markers, is relatively straightforward and apart from blocking of Fc receptors the protocol requires no extra steps. However, when staining intracellular proteins such as cytokines or transcription factors, both fixation and permeabilization steps are required prior to antibody staining.

Updated Flow Cytometry Basics Guide
Practical advice to get you started in flow cytometry.
Introduction to Flow Cytometry Basics
This flow cytometry guide aims to give you a basic overview of all the important aspects of flow cytometry.
With chapters on instrumentation, useful reagents, controls, experimental set-up, and much more, this guide enables best practice to be followed and gives practical advice on building multicolor panels with example protocols.
This guide is invaluable to beginners wanting to start flow cytometry and is a handy tool for teaching others about this powerful application.
To further assist learning we have recently added a flow cytometry glossary of terms to this guide.





